Selected Latest Research Achievements in the Frontiers of Catalytic Materials
1. ACS Nano:Transition metal modified aluminum nanocrystals
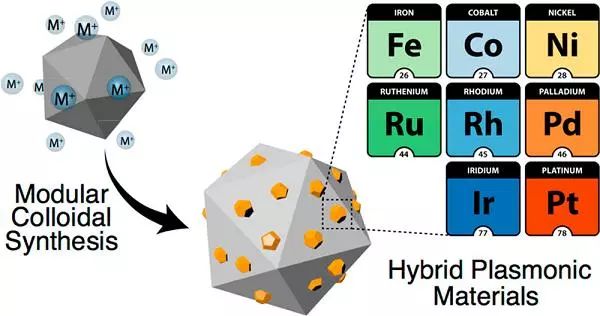
Aluminum with sufficient reserves can serve as a substitute for gold and silver in plasma applications. Aluminum nanocrystals are a promising type of plasma photocatalyst, which exhibit better performance when combined with catalytic metals or oxides to form an antenna reactor heterostructure. Recently, Professor Emilie Ringe and Professor Naomi J. Halas from Rice University in the United States, as well as Professor Rowan K. Leary from the University of Cambridge in the United Kingdom (co corresponding authors), used a simple polyol synthesis route to prepare eight size adjustable transition metal nanoisland modified aluminum nanocrystals, most of which can serve as heterogeneous catalysts. Using scanning transmission electron microscopy and electron tomography to analyze high-resolution three-dimensional structures, the size range of a single nanoisland can reach several nanometers, with a relatively close distance from other nanoislands. When combined with aluminum nanocrystal plasma antenna, the above-mentioned nano islands enhance light absorption and become strong reaction hotspots.
Literature link: Transition-Metal Decorated Aluminum Nanocrystals(ACS Nano, 2017, DOI: 10.1021/acsnano.7b04960)
2. ACS Nano:Intrinsic chirality origin of carbon nanotubes
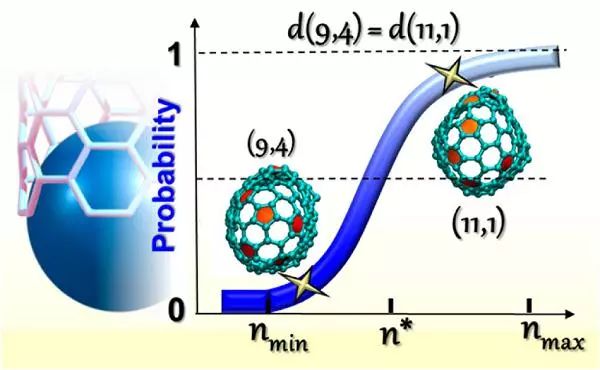
The key to further exploring the potential of carbon nanotubes lies in elucidating their chirality origin. The current popular theory suggests that catalyst structures generate chirality through epitaxial relationships. Researchers such as Avetik R. Harutyunyan from Honda Research Institute in the United States (corresponding authors) compared the chirality abundance of carbon nanotubes grown on floating liquid gallium droplets (without considering the influence of catalyst properties) with those grown on solid Ru nanoparticles. The author believes that the chirality of carbon nanotubes is generally the result of the interaction between the intrinsic tendency of carbon clusters and the catalyst structure. This discovery provides some help for constructing complex nanotube growth theories and lays the foundation for the selective synthesis of carbon nanotubes through liquid catalyst drop chirality.
Literature link: Intrinsic Chirality Origination in Carbon Nanotubes(ACS Nano, 2017, DOI: 10.1021/acsnano.7b03957)
3. ACS Nano:Microwave-assisted Autocatalytic Growth of Nanometals
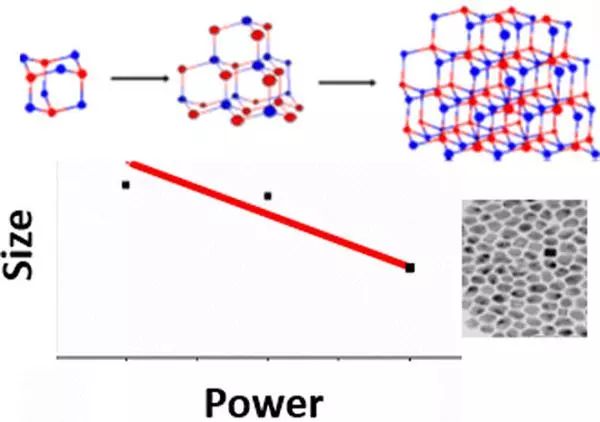
Microwave (MW) synthesis, as a green and sustainable material preparation method, has attracted the attention of many researchers. The reports on improving the formation rate and quality of microwave driven reactions are very attractive, but more attention should be paid to the reaction mechanism and how the above phenomena occur when coupled to the microwave field. Professor Geoffrey F. Strouse (corresponding author) from Florida State University in the United States first used spectroscopic methods to analyze the growth of metal nanoparticles (NPs) in microwaves, and compared them with classical methods of NP self catalytic growth, thereby elucidating the basis for the observed enhanced growth behavior of metal NPs prepared in microwave fields. The study clearly indicates that microwave growth is modeled by the self catalytic mechanism, which has led to a widely reported increase in formation rate and quality improvement in nanomaterial literature.
Literature link: Microwave Enhancement of Autocatalytic Growth of Nanometals(ACS Nano, 2017, DOI: 10.1021/acsnano.7b04040)
4. Adv. Energy Mater.:
Palladium nanoparticle loaded rutile TiO2 black phosphorus composite material with heterogeneous interface: high electrocatalytic activity and durability catalyst for ethanol electrocatalytic oxidation
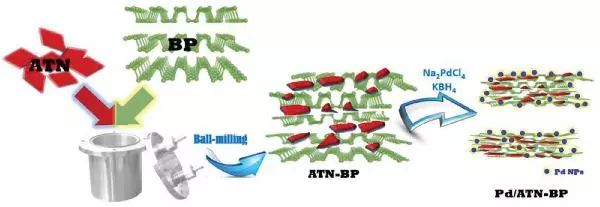
Designing high-performance palladium (Pd) substrates with enhanced ethanol oxidation reaction (EOR) activity has always been a challenge. Professor Min Yulin, Professor Xu Qunjie, and Associate Professor Fan Jinchen (co corresponding authors) from Shanghai Electric Power University have prepared a new type of rutile TiO2 nanosheets black phosphorus (ATN-BP) composite as the substrate for Pd nanoparticles, and used it for EOR. Under argon protection, BP nanosheets were directly ball milled with ATN to form ATN-BP composite materials, where BP nanosheets were interconnected with fragmented ATN through P-O-Ti bonds. The ATN-BP structure is not only beneficial for improving electrolyte penetration and electron transport, but also has a strong impact on the synergistic interaction between Pd and ATN-BP to remove active intermediates. The results indicate that Pd/ATN-BP composite materials with heterogeneous interfaces of Pd, BP, and ATN exhibit ultra-high electrocatalytic activity and durability.
Literature link: Palladium Nanoparticles Anchored on Anatase Titanium Dioxide-Black Phosphorus Hybrids with Heterointerfaces: Highly Electroactive and Durable Catalysts for Ethanol Electrooxidation(Adv. Energy Mater. , 2017, DOI: 10.1002/aenm.201701799)
5. Adv. Energy Mater.:Adjusting the catalytic performance of CO2 hydrogenation through thermoelectric materials
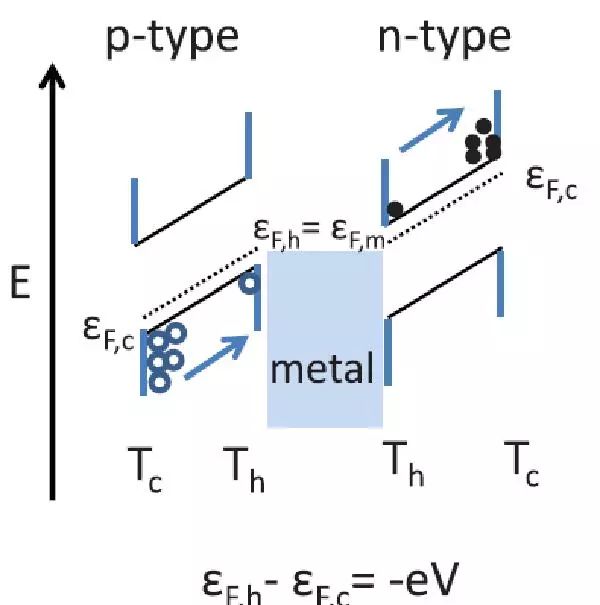
Thermoelectric (TE) materials have recently attracted widespread research interest. Most of the TE materials currently used are heavy metal based materials, such as Bi2Te3, PbTe, etc. However, the above-mentioned materials are not suitable for large-scale applications as media at high temperatures, as their melting point, decomposition or oxidation temperature are relatively low. Zhaorong Huang (corresponding author) and others from the University of Cranfield in the UK have innovatively utilized the thermoelectric material BiCuSeO as a substrate to promote catalytic hydrogenation of carbon dioxide. Thermoelectric materials have the ability to move the Fermi level and work function of catalysts, resulting in an exponential increase in catalytic activity of catalyst particles deposited on their surfaces. The experimental results indicate that the significant increase in CO2 conversion rate and selectivity is related to the Seebeck voltage of thermoelectric. This indicates that the thermoelectric effect not only increases the reaction rate, but also shifts the chemical equilibrium, leading to thermodynamic changes in CO2 conversion during hydrogenation reactions.
Literature link: Tuning of Catalytic Activity by Thermoelectric Materials for Carbon Dioxide Hydrogenation(Adv. Energy Mater., 2017, DOI: 10.1002/aenm.201701430)
6. Adv. Funct. Mater.:Multi functional Mo-N/C @ MoS2 electrocatalysts for HER, OER, ORR, and zinc air batteries
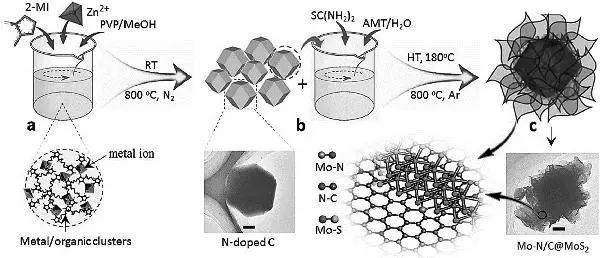
Replacing precious metal platinum catalysts with inexpensive, stable, and efficient electrocatalysts has enormous potential in the application of large-scale clean energy devices. Metal organic frameworks (MOFs) and metal sulfides (MDs) provide a broad platform for the design of highly active electrocatalysts due to their ultra-high surface area, layered pore structure, and high catalytic activity. Professor Mu Shichun (corresponding author) and others from Wuhan University of Technology prepared a composite electrocatalyst based on MoS2 nanosheets vertically coated with Mo-N coupling centers at the interface in the Mo-N/C framework. The above composite materials exhibit good electrocatalytic activity and stability in hydrogen evolution reaction (HER), oxygen evolution reaction (OER), and oxygen reduction reaction (ORR), making them a multifunctional electrocatalyst. Interestingly, the electrocatalyst also exhibits high performance as a cathode for zinc air batteries. The excellent electrochemical activity can be attributed to the synergistic effects between different chemical components, unique three-phase active sites, and the rapid mass transport brought about by the layered pore framework. This work is expected to provide inspiration for designing leading and efficient MOF/MD based composite electrocatalysts for electrochemical energy devices.
Literature link:Multifunctional Mo–N/C@MoS2 Electrocatalysts for HER, OER, ORR, and Zn-Air Batteries(Adv. Funct. Mater. , 2017, DOI: 10.1002/adfm.201702300)
7. Adv. Funct. Mater.:Ordered Superparticles with Enhanced Photoelectric Effect
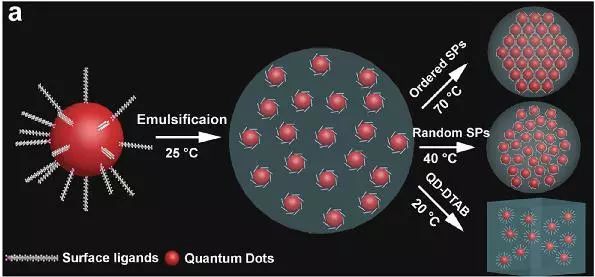
The surface of nanocrystals is generally passivated by insulating organic ligands, but organic ligands hinder electron transfer between adjacent modules of self-assembled nanoparticles. Ligand excision or exchange is a feasible strategy to promote electron transfer, but often surface state changes can lead to performance degradation or uncontrollable aggregation. Professor Wang Tie, Institute of Chemistry, Chinese Academy of Sciences (corresponding author) and others prepared 3D super compact super particles with well-defined superlattice domains by using thermally controlled lotion based self-assembly method. The particle spacing in the superparticle is reduced to about 0.3 nm, as the organic ligand crawls on the surface of the nanoparticle, which is sufficient to overcome the obstacle of electron transfer. The ordered and compact superstructure promotes coupling and electron energy transfer between CdSSe quantum dots (QDs).
Literature link: Ordered Superparticles with an Enhanced Photoelectric Effect by Sub-Nanometer Interparticle Distance(Adv. Funct. Mater. , 2017, DOI: 10.1002/adfm.201701982)
8. Energy Environ. Sci.:Application of MoS2/TiO2 plasma photocatalyst in efficient hydrogen production

Recently, Professor Yang Yang from the University of Central Florida in the United States and Senior Researcher Du Yingge from the Pacific Northwest National Laboratory (co corresponding author) used electrochemical corrosion to prepare ordered TiO2 nanotube array thin films. Then, multiple layers of molybdenum sulfide (MoS2) nanosheets were uniformly grown on the inner surface of TiO2 nanotubes and applied to solar water splitting for hydrogen production. Through experiments and theoretical calculations, it has been proven that sulfur defects in MoS2 are the cause of visible light absorption in this material. Combining the UV absorption of TiO2 and the near-infrared absorption of MoS2 itself, a catalyst with broad spectral absorption has been prepared. TiO2 nanotube arrays not only absorb ultraviolet light, but also serve as the substrate for MoS2 catalysts, thereby improving the efficiency of solar light utilization and the stability of photocatalytic processes. Further experiments have shown that the above catalysts can achieve hydrogen production by decomposing seawater.
Literature link: MoS2/TiO2 heterostructures as nonmetal plasmonic photocatalysts for highly efficient hydrogen evolution(Energy Environ. Sci., 2017, DOI: 10.1039/c7ee02464a)
9. Energy Environ. Sci.:W1-xIrxO3 synthesized by plasma with low content of precious metals- δ Water oxidation electrocatalyst

Using renewable electricity to decompose water may be one of the most promising ways to sustainably produce hydrogen. At present, commercialized water oxidation catalysts still mainly rely on expensive and rare precious metal oxides. Joshua M. Spurgeon (corresponding author) from the University of Louisville in the United States used abundant tungsten as a structural metal to reduce the use of precious metal iridium while maintaining high activity and stability. As the metal Ir content drops to 1%, the overpotential of oxygen evolution has become somewhat competitive. Compared to iridium polytungstate with higher Ir content, iridium polytungstate with higher crystallinity and pure phase has better catalytic activity at lower content. In addition, the electrocatalytic performance of materials synthesized by plasma method is significantly improved compared to those synthesized by standard thermal oxidation, demonstrating the significance of non-equilibrium synthesis for discovering new catalysts.
Literature link: A low-noble-metal W1-xIrxO3-δ water oxidation electrocatalyst for acidic media via rapid plasma synthesis(Energy Environ. Sci., 2017, DOI: 10.1039/c7ee02626a)
10. Nano Energy:Composition adjustable Au Pt bimetallic thin film for electrocatalytic reduction of CO2
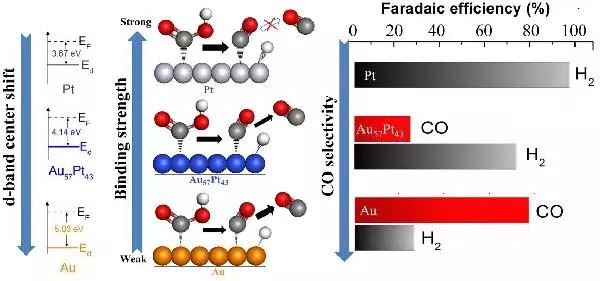
Au electrocatalysts can reduce CO2 to CO, but the catalytic activity of Au is still limited by CO2 activation. On the contrary, Pt can activate CO2 and convert it into adsorbed state CO. Wilson A. Smith and colleagues from Delft University of Technology in the Netherlands first investigated the electrocatalytic reduction performance of Au Pt bimetallic thin films with controllable composition and planar morphology for CO2. The influence mechanism of the composition of binary catalysts on the catalytic activity and selectivity for reducing CO2 was systematically studied through experiments and theories. The formation of the main products (H2 and CO) is closely related to the changes in surface composition, while the formation of HCOOH occurs at lower potentials, resulting in a significant increase in yield. Therefore, the surface composition and bimetallic synergistic effect of the two metals contribute to the overall performance improvement of Au Pt electrocatalysts for CO2 reduction.
Literature link:Electrochemical Reduction of CO2 on Compositionally Variant Au-Pt Bimetallic Thin Films(Nano Energy, 2017, DOI: 10.1016/j.nanoen.2017.09.043)
11. Nature Energy:Introducing N atoms into Co nanosheets to enhance CO2 hydrogenation activity

Converting CO2 into fuel and useful chemicals through hydrogenation can help reduce dependence on fossil fuels. In the past few decades, although significant progress has been made, there are still significant challenges in improving the activity of CO2 hydrogenation catalysts and developing efficient catalysts based on non precious metals. Professor Zeng Jie (corresponding author) from the University of Science and Technology of China introduced N atoms into cobalt nanosheets to enhance CO2 hydrogenation activity. The reversal frequency of Co4N nanosheets in CO2 hydrogenation reaction is 25.6 h-1, which is 64 times that of Co nanosheets. The activation energy of Co4N nanosheets is 43.3 kJ · mol-1, which is less than half of that of cobalt nanosheets. Mechanism studies have shown that Co4N nanosheets are converted into Co4NHx, where the amino hydrogen atom directly forms an intermediate HCOO * with CO2. In addition, the adsorbed H2O * activates amino hydrogen atoms through hydrogen bonding interactions.
- Prev:没有了!
- Next:Wire harness for electrically heated catalysts

